When winter arrives, we all prepare our heating system which includes an electric heater, coal heaters, or gas heater. These heaters provide us heat in winter so we can warm up our bodies. Also, we use heat for cooking our food. Sometimes excess heat disturbs our living, so air-conditioners are used to remove that excess heat.
What is heat?
Heat is actually a kind of energy transfer from one object to another, when there is a temperature difference between them. Heat always flow from hot object to cold object. Don’t confuse yourself with heat and temperature, they both are separate quantities. Heat is measured joule; the unit in which energy is measured. Whereas, temperature tells, how hot a substance is?
Heat is measured in joules; the same unit of energy measurement. There are other units for measuring heat, which are BTU (British Thermal Unit) and calorie.
Heat transfer
Heat can be transferred in several ways. There are three methods in which heat can be transferred. These methods are:
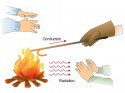
- Conduction: Conduction takes place when two objects are in contact with each other.
- Convection: In this methods, heat is transferred by flow and diffusion of liquids from high-temperature regions towards low-temperature
- Radiation: In this method, heat is transferred by radiation e.g. visible light and infrared rays.
Read more about Heat transfer in our article: Heat Transfer
Effect of heat on states of the matter
As the heat of an object increase, its temperature also increases. Each type of substance has a certain value to remain in its particular state (solid, liquid, or gas). When temperature increases or decreases from these values the state of the substance is changed. For example, water turns into ice when the temperature drops below the 0 ⁰C. When the temperature reaches 100 ⁰C, the water starts to boil and is converted into steam (gaseous state of water).
Note: Pressure of the atmosphere also affects the state of the substance.
Facts
- A substance containing more heat does not always mean that its temperature is also high.
- A cup of tea at 50 ⁰C has a lot more heat than a drop of water at 99 ⁰
- Each material has a certain capacity to hold the heat at a particular temperature. It is called the heat capacity.
- A cup of water at 99 ⁰C will not warm up the bucket of water. But, another half filled bucket at 60 ⁰C can warm up the water in the first It is because half filled bucket has more heat than the cup of water at 99 ⁰C.