Tag: physics
-

Radioactivity
Radioactivity is a very famous term in nuclear physics and chemistry that describes how unstable atoms release certain radiations for the purpose of getting stability. You may have probably heard about uranium, a radioactive element, used for making atomic bombs and electricity from nuclear power plants. It is possible due […]
-

Lasers
Do you remember the movies where alien forces use laser guns in battles? Well, lasers are a very famous piece of technology. These were once considered as high-tech gadgets, but today modern science made lasers very common technology used in everyday life. What is LASER? A laser is just a […]
-

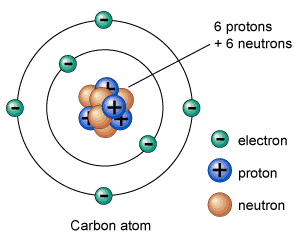
What is Atom
Atom is the smallest building block of an element – it can’t be further broken by any chemical process. Everything you see around yourself is made of trillions-of-trillions atoms. Atoms are so small that, it is not possible to see them with a powerful microscope. Only specialized microscopes (electron microscope) […]
-

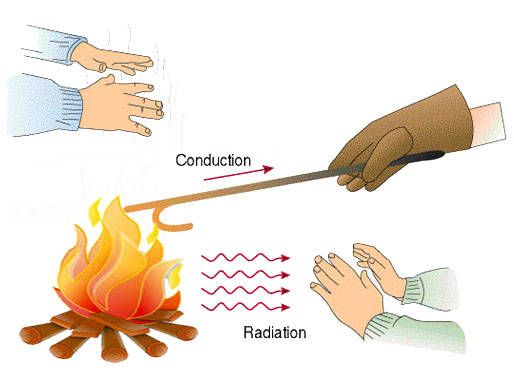
Heat Transfer
Heat transfer is the transfer of thermal energy from one object to another. It occurs between two objects when they are at different temperatures – the heat will transfer from hot object to cold object. In our environment heat transfer occurs all the time. The sun is transferring some of […]
-