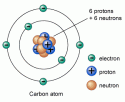
Radioactivity is a very famous term in nuclear physics and chemistry that describes how unstable atoms release certain radiations for the purpose of getting stability. You may have probably heard about Uranium, a radioactive element, used for making atomic bombs and electricity from nuclear power plants. It is possible due to the radioactive nature of uranium.
Radioactivity is a very famous term in nuclear physics and chemistry that describes how unstable atoms release certain radiations for the purpose of getting stability. You may have probably heard about Uranium, a radioactive element, used for making atomic bombs and electricity from nuclear power plants. It is possible due to the radioactive nature of uranium.
What is Radioactivity?
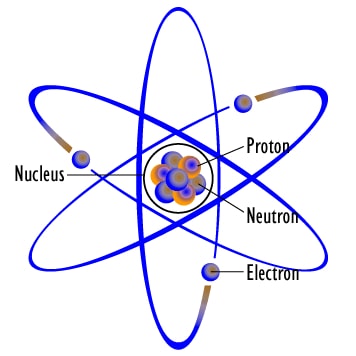
Radioactivity is a natural phenomenon in which atoms of Elements that have unstable nuclei (nuclei = plural of nucleus), disintegrate for getting stability. There are 3 main reasons behind an unstable nucleus in an atom. These reasons are:
- higher number of protons
- higher number of neutrons
- higher energy of the nucleus
When an element decay, it can change from one element to another for achieving stability. Sometimes when an element decay, it doesn’t change from one element to others but simply gets stability by releasing extra Energy in the form of gamma rays.
Isotopes
There are around 118 known elements that differ from each other by the number of protons in their atoms. For example, hydrogen has 1 proton and Helium has 2 protons. The nucleus also has neutrons which when change in an element, creates a new isotope of that element.
Isotopes of an element have the same chemical properties, but they differ only in weight due to a difference in the number of neutrons. For example, Hydrogen has three isotopes; protium, deuterium, tritium. These isotopes differ from each other due to a difference in the number of neutrons, while the number of protons remains the same. Elements from hydrogen to bismuth in the periodic table have at least one stable isotope. But, elements after bismuth have no stable isotope; they are all radioactive.
Types of Radioactivity
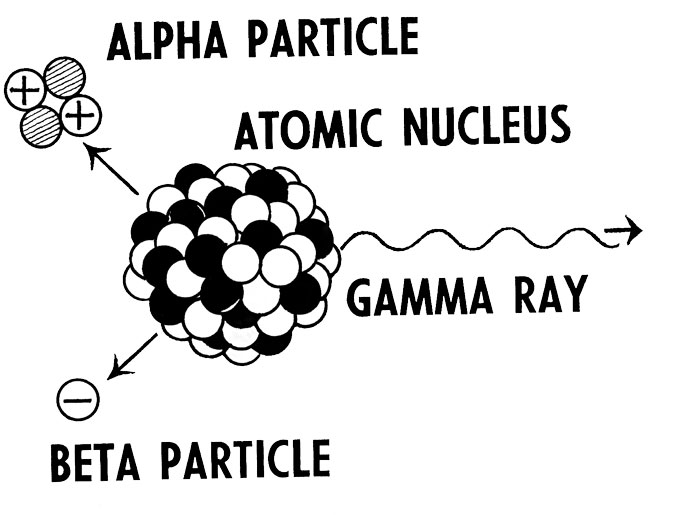 Radioactive isotopes of elements can emit 3 three types of radiations known as alpha rays, beta rays, and gamma rays.
Radioactive isotopes of elements can emit 3 three types of radiations known as alpha rays, beta rays, and gamma rays.
- Alpha rays – Alpha rays are positive charged particles that are composed of two protons and two neutrons (also called nuclei of helium). Alpha rays have a positive charge due to the presence of protons; the positively charged particles.
- Beta rays – Beta rays are the streams of negatively charged particles, e.g. electrons, or positrons.
- Gamma rays – Gamma rays are neutral and are different from alpha rays and beta rays because they are not made of matter particles. Instead, they are high energy electromagnetic radiations; a form of light that is very energetic and can’t be seen with naked eyes.
Half-Life and Measurement of Radioactivity
A sample of a radioactive isotope of an element decays continuously at a fixed rate until the whole material has change into a stable isotope of an element. The term half-life is often used to define time in which half of the sample will turn into stable isotope. For example, the half-life of carbon-14 is around 5,730 years. It defines, if you place 1 kg sample of carbon-14, half of the carbon-14 will turn into a stable isotope in around 5,730 years.
Radioactivity and Human Health
Radiations from the decay of radioactive isotopes are not considered good for health due to several dangers involved in it. For example, when these radiations act on the human body, it damages the DNA and structures of cells. This results in the production of cancerous cells in our body and ultimately make a person very sick.
Facts
- Radioactivity was first discovered by Henry Becquerel while working on an ore of uranium element called pitchblende.
- Not all radiations are bad! We use X-rays to take photographs of the human body’s inside for detecting any damage or diagnosing some diseases.
- Gamma rays are very penetrating radiations, they can easily penetrate through 1 meter thick wall of concrete – much like visible light penetrates through a transparent glass.